FDA Warns the Public Against Purchasing the Following Unregistered Medicines
The Food and Drug Administration (FDA), through four separate advisories published on January 13, 2021, has warned all healthcare professionals and the public against purchasing and using twenty (20) unregistered medicines. Most of which were labeled in a foreign language.
Furthermore, since the FDA Post-Marketing Surveillance (PMS) activities have verified that the products have not gone through the registration process and have not been issued with proper authorization, the agency bared that it cannot guarantee the products’ quality, safety, and efficacy.
“Napatunayan sa pamamagitan ng isinagawang Post-Marketing Surveillance (PMS) ng FDA na nag mga nasabing gamot ay hindi dumaan sa proseso ng rehistrasyon ng Ahensya at hindi nabigyan ng kaukulang awtorisasyon tulad ng Certificate of Product Registratin (CPR). Dahil dito, hindi masisigurado ng Ahensya ang kalidad, kaligtasan at bisa nito.”
“Therefore, consumption of such violative products may pose potential danger or injury to your health,” added the agency.
Unregistered Medicines
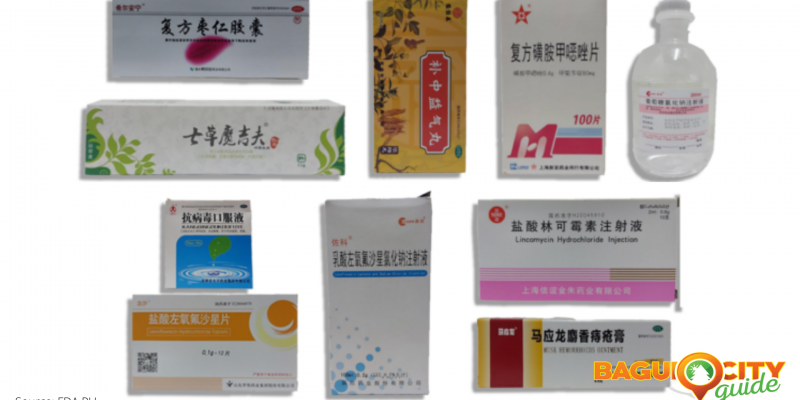
Here are the brands/names and photos of the following unregistered medicines the Food and Drug Administration has warned the public about:
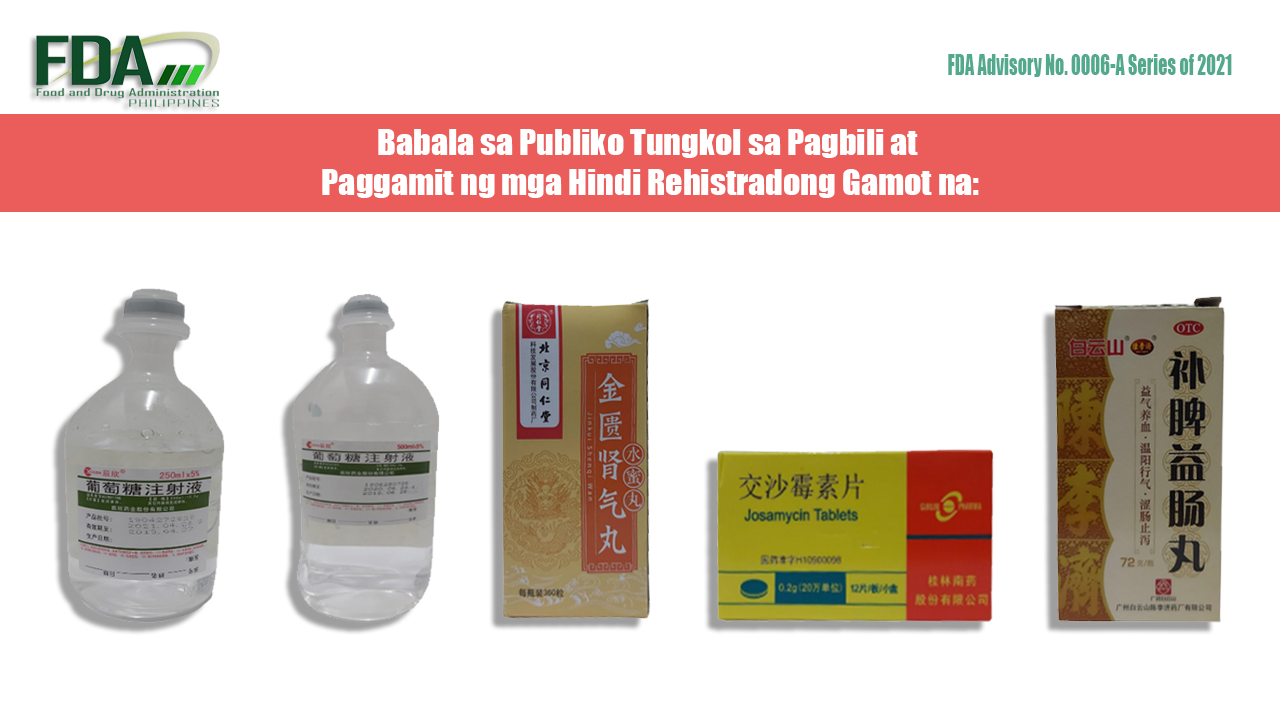
Unregistered Medicines from FDA Advisory No. 0006-A Series of 2021
- CISEN® H37021756 5% 250ml 12.5g [Label in Foreign Language]
- CISEN® H37021825 5% 500ml:25g [Label in Foreign Language]
- Jinkui Shenqi Wan
- Josamycin Tablets 0.2g
- OTC Z44022627 [Label in Foreign Language]
Unregistered Medicines from FDA Advisory No. 0007-A Series of 2021
- OTC Bei® Kangbingdukoufuye
- Levofloxacin Hydrochloride Tablets 0.1g
- CISEN® Levofloxacin Lactate and Sodium Chloride Injection 100ml:0.2g
- SINE® Lincomycin Hydrochloride Injection 2ml : 0.6g
- OTC Musk Hemorrhoids Ointment
Unregistered Medicines from FDA Advisory No. 0008-A Series of 2021
- OTC Guo Guang® Z50020601 [Label in Foreign Language]
- OTC Z34020127 [Label in Foreign Language]
- SPH H31020387 [Label in Foreign Language]
- GB15979 12g [Label in Foreign Language]
- CISEN® H37021822 250ml [Label in Foreign Language]
Unregistered Medicines from FDA Advisory No. 0009-A Series of 2021
- A Qi Mei Su Fen San Pian
- OTC Anshenbunaoye
- Erythromycin Estolate Tablets
- OTC Huoxiang Zhengqi Shui
- OTC [Label in Foreign Language]
Related
FDA warns the public not to use the following unnotified face masks. Click, HERE.
For More News and Updates
Looking for more news and updates? Feel free to explore our BCG website and our official Facebook page. You may also check out our official BCG YouTube channel to catch a variety of video content.
Source: FDA Advisory No.2021-0006-A, FDA Advisory No.2021-0007, FDA Advisory No.2021-0008-A, FDA Advisory No.2021-0009-A