FDA Warns Public Not to Purchase the Following Products
In separate advisories, the Food and Drug Administration (FDA) – Philippines warned the public not to purchase and use the following products:
FDA Advisory No.2206, Series of 2020
- Collagen by Watsons Moisturising & Brightening Facial Mask

Photo Source: FDA
In the advisory, FDA has verified through postmarketing surveillance and it showed that the said product has no valid Certificate of Product Notification (CPN) as of December 15, 2020.
As stated in the advisory, under the FDA Act of 2009, the following is prohibited without the proper authorization from the FDA:
- manufacture
- importation
- exportation
- sale,
- offering for sale
- distribution
- transfer
- non-consumer use
- promotion
- advertising
- sponsorship of any health
FDA Advisory No. 2193, Series of 2020
- IWHITE Korea Matcha Ice Cream Wash Off Mask

Photo Source: FDA
The cosmetic product’s non-compliance is that the ingredients listed on the label are inconsistent with the information declared in the acknowledged product notification according to the FDA advisory.
Furthermore, FDA has verified the product as Non-compliant through post-marketing surveillance.
FDA stated on the advisory that “based on the CPN issued to the company, any subsequent changes to the information previously submitted to FDA will render the notification invalid.”
Also, the FDA added that “all concerned establishments are warned not to distribute violative product until they have fully complied with the rules and regulations of the FDA.”
FDA Advisory No. 2184, Series of 2020
- Hisamitsu® Bye-bye Fever (For Adults)
- Hisamitsu® Bye-bye Fever (For Babies)

Source: FDA
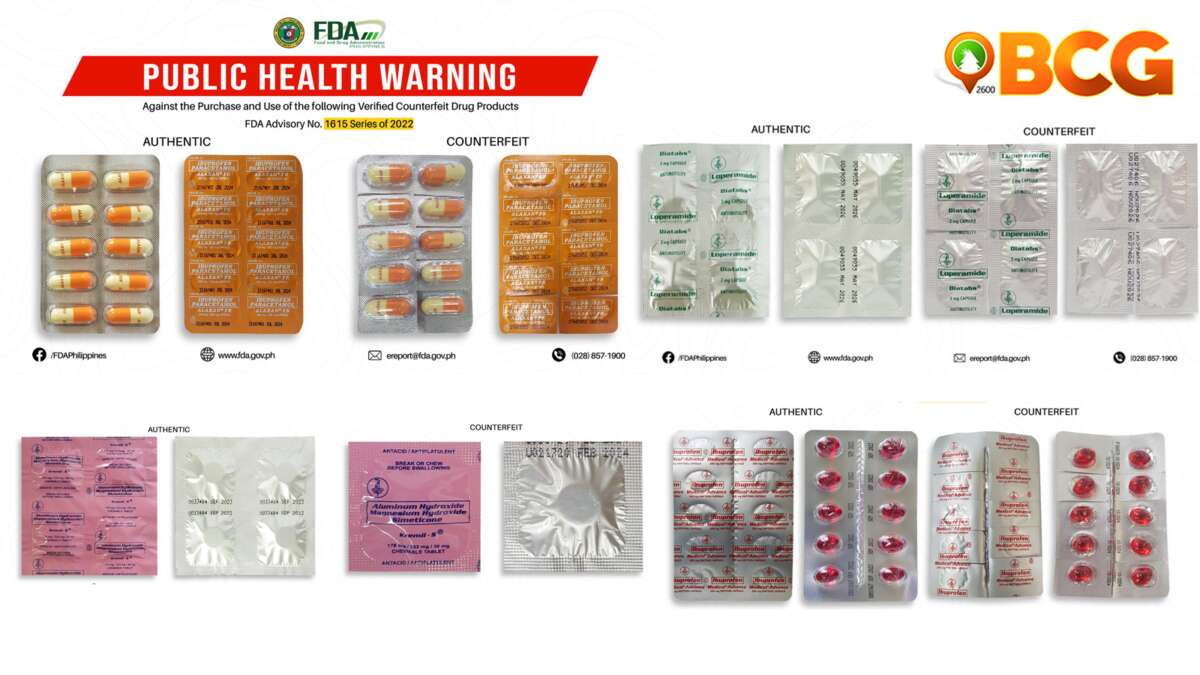
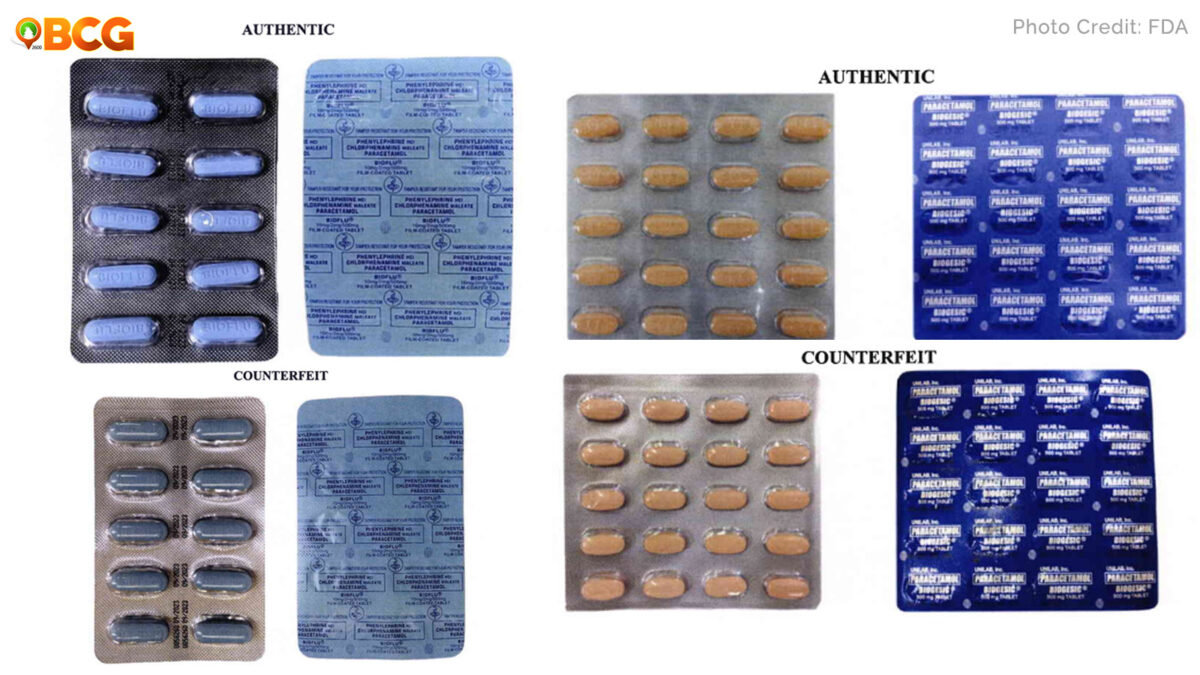
Through post-marketing surveillance, FDA verified that the said products are not “not notified and no corresponding Product Notification Certificates have been issued.”
In addition, the quality and safety of the abovementioned medical products cannot be assured since it has not gone through the evaluation process of FDA.
FDA Advisory No.2188 and FDA Advisory No.2187
Under the FDA Advisories, the public is warned not to purchase and use the following unauthorized cosmetic products:
- POUF! EVERYDAY BLOOM COLOGNE SPRAY
- POUF! EVERYDAY DUSK COLOGNE SPRAY

Photo Source: FDA

Photo Source: FDA
According to the advisory, the said products were “verified by FDA through postmarketing surveillance and shows that no valid CPN as of December 17, 2020.
As stated in the advisory, since the unauthorized products “has not gone through the notification of process of the FDA, the agency cannot assure their quality and safety. The use of such violative product may pose health risks to consumers.”
Report Sale/Distribution
To report any sale or distribution of unnotified medical device products, non-compliant cosmetic products, and unauthorized cosmetic products, report through the online reporting facility eReport.
For More News and Updates
Looking for more news and updates? Feel free to explore our BCG website and our official Facebook page. You may also check out our official BCG YouTube channel to catch a variety of video content.
Source: FDA Advisory No.2206, FDA Advisory No. 2193, FDA Advisory No. 2184, FDA Advisory No.2188, FDA Advisory No.2187