FDA Warns Public Not to Purchase the Following Counterfeit and Non-Compliant Products
The Food and Drug Administration (FDA) has released advisories warning the public from purchasing and using the verified counterfeit drug and non-compliant cosmetic products.
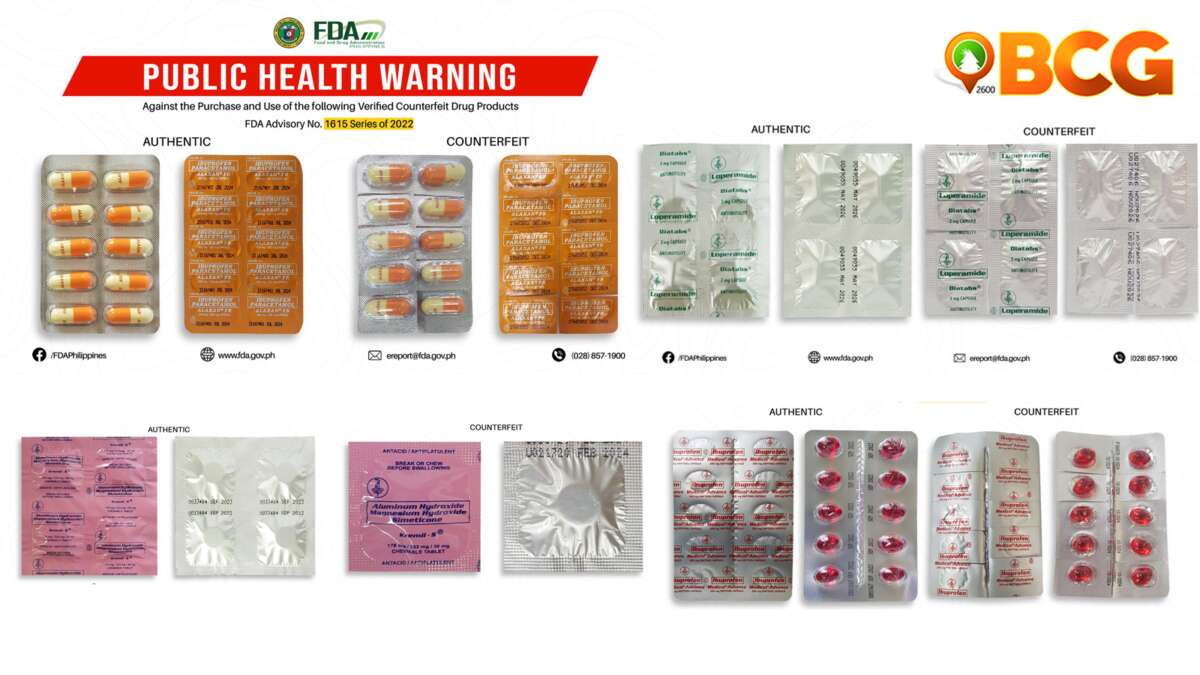
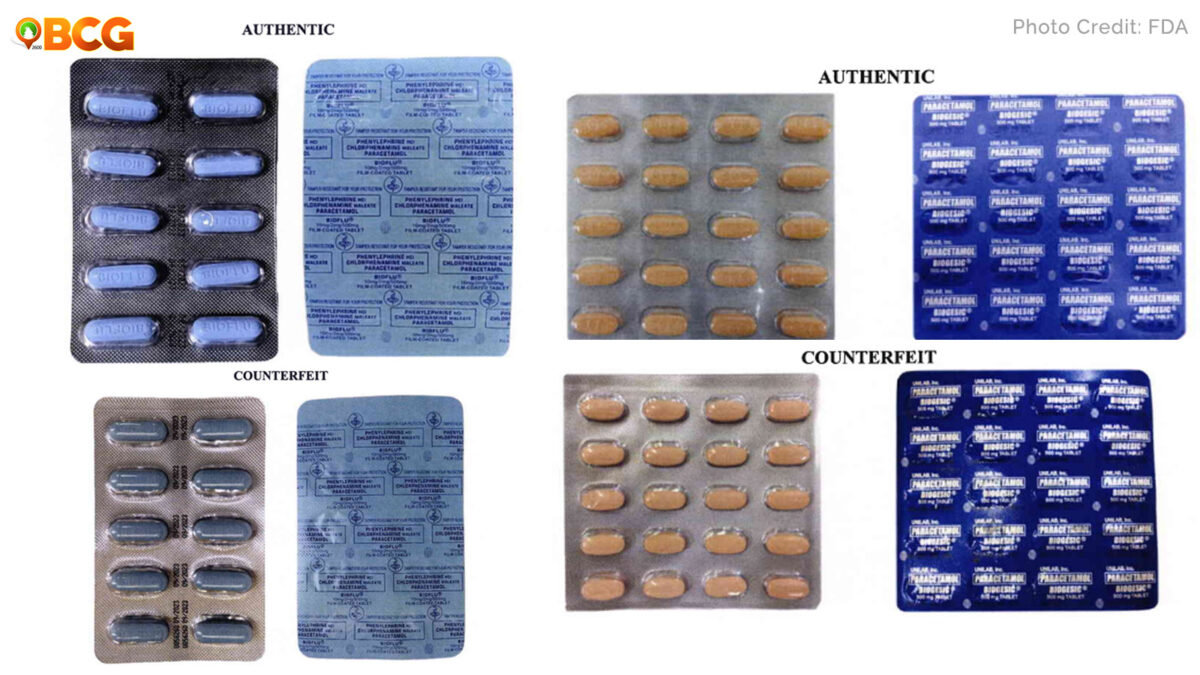
FDA Advisory No. 2020-1877
- Mefenamic Acid (Ponstan®) 500mg Tablet
- Loperamide (Diatabs®) 2mg Capsule
- Carbocisteine (Solmux®) 500mg Capsule
- Phenylephrine HCl / Chlorphenamine Maleate / Paracetamol (Neozep® Forte) 10mg/2mg/500mg Tablet
“All healthcare professionals and the general public are hereby warned as to the availability of these conterfeit drug products in the market which pose potential danger or injury to consumers. Consumers are also reminded to purchase drug products only from FDA-licenses establishments.”
-FDA Advisory
FDA Advisory No. 2020-1982
According to the FDA Advisory No. 2020-1982, the public is warned from purchasing and using the said non-compliant cosmetic product:
- PHCare Intimate Wash Coolwind With Active Cool
It was stated in the agency’s advisory that the aforementioned product has failed to declare on the label the full ingredient list.
“The Food and Drug Administration has verified that the abovementioned product is NON-Compliant through its postmarketing surveillance (PMS) pursuant to Book I, Article II, Section 2 of the Rules and Regulations Implementing Republic Act No. 9711, otherwise known as the “Food and Drug Administration Act of 2009″ which provided for the relevant functions, powers and duties of the agency, including the conduct of PMS activities in the monitoring of health products.”
-FDA Advisory
Report
Furthermore, the FDA has advised the public to report the continuous sale or distribution of unregistered health and violative cosmetic products through eReport or call the Center for Drug Regulation and Research at (02) 8809-5596.
Source: FDA Advisory No. 2020-1982, FDA ADVISORY No. 2020-1877